Chimeric Genes
Chimeric genes arise when portions of two existing coding sequences combine to produce a single open reading frame. These new genes can provide a significant source of genetic novelty and are often associated with selective sweeps.
Formation
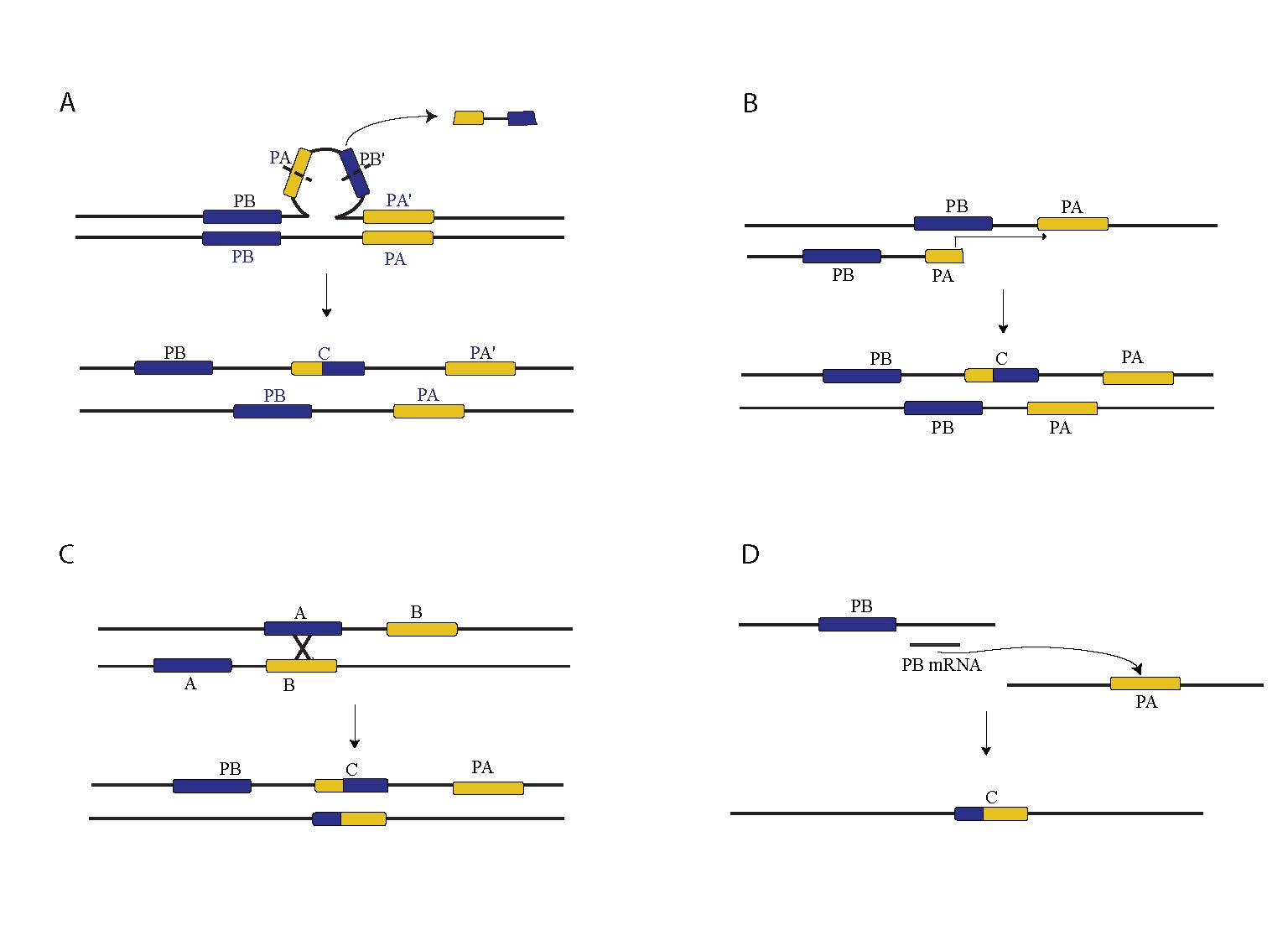 Figure 1. Mechanisms of Chimeric Gene Formation. A. Large loop mismatch repair. B. Replication Slippage. C. Ectopic Recombination. D. Retrotransposition.
Figure 1. Mechanisms of Chimeric Gene Formation. A. Large loop mismatch repair. B. Replication Slippage. C. Ectopic Recombination. D. Retrotransposition.
In D. melanogaster chimeric genes are most likely to form via tandem duplications that have not respected gene boundaries. Such constructs may arise through two molecular mechanisms, one which is similar to replication slippage but which operates over longer distances, and the other which invokes the action of the large loop mismatch repair system (Fig. 1A, 1B). In other organisms, including humans, chimeric genes have also formed through ectopic recombination as well as through retrotransposition of coding sequences, likely due to higher repetitive DNA content and higher rates of transposable element activity (Fig 1C, 1D).
Our current work in D. yakuba suggests that an appreciable number of chimeric genes form at the boundaries of chromosomal rearrangements, in contrast with chimeric genes identified in the D. melanogaster reference genome. The causes of this disparity are not yet clear, though it is possible that repetitive elements play a strong role.
Selection
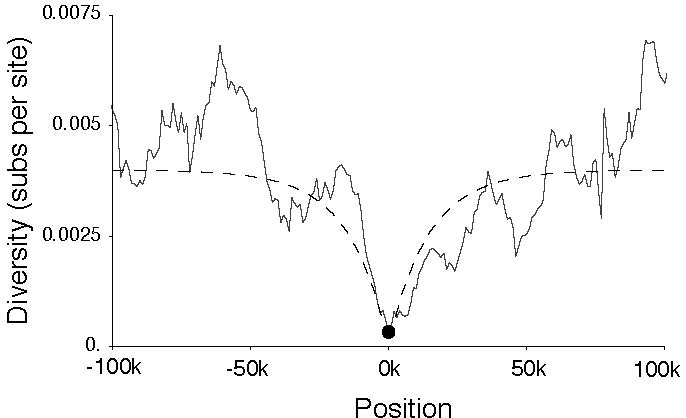 Figure 2. Selective Sweep on Qtzl.
Figure 2. Selective Sweep on Qtzl.
Chimeric genes are often the targets of positive selection, and can spread through populations quickly after formation. Several young chimeric genes in D. melanogaster are associated with selective sweeps, and young chimeric genes are far more likely to be adaptive than duplicate genes of the same age.
Quetzalcoatl is one example of a young chimeric gene which has experienced a recent selective sweep around 15,000 years ago (Fig. 2). Shortly after formation, Qtzl spread through the population, resulting in reduced nucleotide diversity and highly skewed site frequency spectra in the genomic region surrounding the locus. This chimeric gene has been found in every strain of D. melanogaster surveyed to date, but has not been found in strains of
Outside D.melanogaster several other chimeric genes have been identified which show signs of rapid protein evolution, consistent with natural selection. The majority of these genes in Drosophila are derived from Adh coding sequences.
Gene Regulation
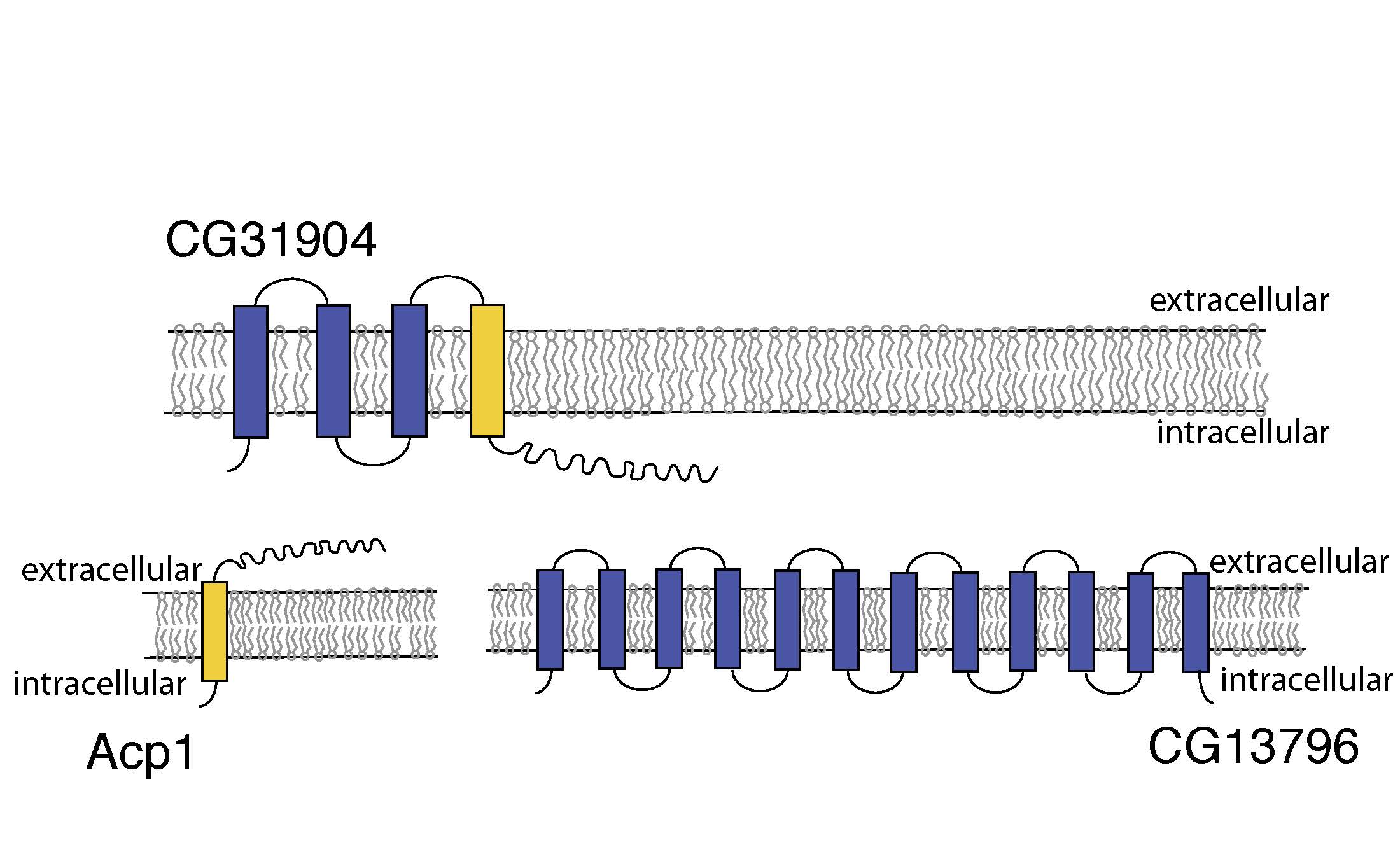 Figure 3. Altered transmembrane orientation of CG31904.
Figure 3. Altered transmembrane orientation of CG31904.
Through the combination of different regulatory domains derived from each of the parental genes, chimeric genes can cause changes in when and where newly formed coding sequences are expressed. Even DNA-level tandem duplications can create chimeric genes whose regulatory profiles differ from one or more parental genes.
Additionally, chimeric genes can rearrange protein domains, altering the cellular localization of various proteins. Chimeras can place a peptide under the control of a new mitochondrial or secretory target peptide, or may alter the the transmembrane status of a protein.
Acp1 is a transmembrane cuticular peptide which contains multiple transmembrane domains. CG31904 unites a portion of Acp1 with additional transmembrane helices, therby altering the transmembrane orientation of the peptide (Fig. 3). This ability of chimeric genes to alter gene expression patterns, cellular localization, and transmembrane orientation offers additional axes whereby chimeric genes can generate variation and may have a strong influence on their role as a source of functional novelty.
We will examine the chimeric genes we have identified in D. yakuba to determine whether they follow similar patterns to those observed in D. melanogaster, esepcially with regards to how different mechanisms of formation might influence their impacts on expression levels and the likelihood that they are involved in adaptive evolution.